February 2021
Real Regulatory Tips and Insights

The tips we published in February 2021 are collected here, for convenience. Make sure you follow Real Regulatory Ltd and Real CMC for regulatory news, reports and hints.
Clinical Trials Facilitation Group (CTFG) Guidance for Clinical Trials

Orphan Condition & Orphan Indication in the European Union

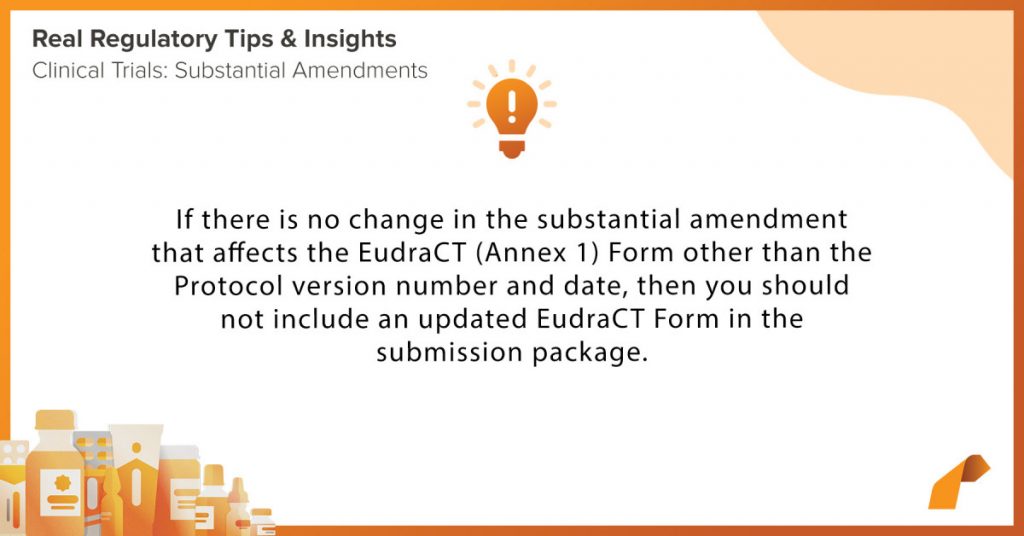
Clinical Trials: Substantial Amendments
Clinical Trials: Planning for EU Sites

For previous monthly round-ups, you can follow these links:
January 2021 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/january-2021-real-regulatory-tips-and-insights/
December 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/december-2020-real-regulatory-tips-and-insights/
November 2020 Real Regulatory Tips & Insights Round-Up: https://www.realregulatory.com/november-2020-real-regulatory-tips-and-insights/

